Embark on a journey into the fascinating world of ionic compounds with our comprehensive formation of ionic compounds worksheet. This interactive tool delves into the intricacies of ionic bond formation, providing a thorough understanding of their properties, applications, and safety considerations.
As you navigate through this worksheet, you will discover the fundamental principles governing the formation of ionic compounds, their unique characteristics, and their indispensable role in various industries. Prepare to unravel the secrets of these remarkable substances that shape our world.
Introduction
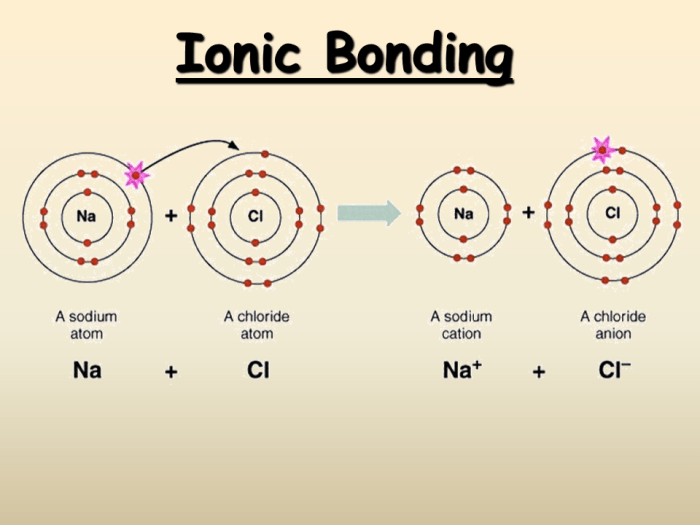
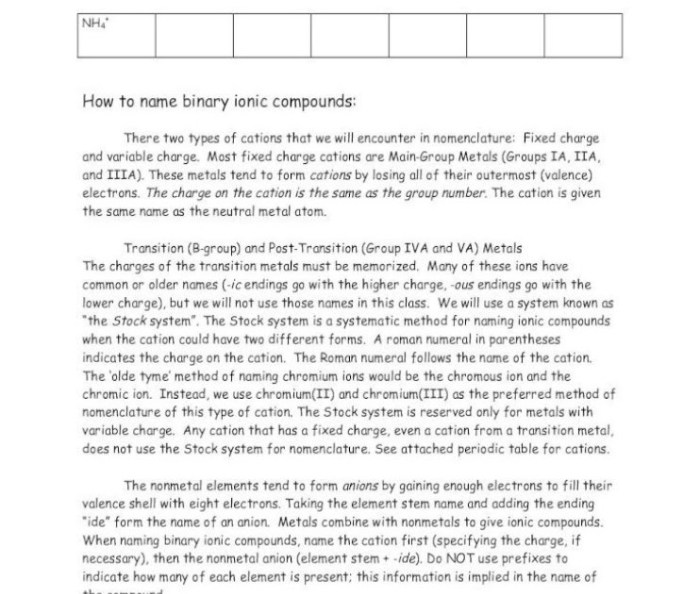
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The metal becomes a positively charged ion, called a cation, and the nonmetal becomes a negatively charged ion, called an anion. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Formation Process of Ionic Compounds
The formation of ionic compounds can be represented by a chemical equation. For example, the formation of sodium chloride (NaCl) can be represented by the following equation:
Na + Cl2→ 2 NaCl
In this equation, two atoms of sodium (Na) react with one molecule of chlorine (Cl 2) to form two molecules of sodium chloride (NaCl). The sodium atoms lose one electron each, becoming sodium ions (Na +), and the chlorine atoms gain one electron each, becoming chloride ions (Cl –). The oppositely charged ions are attracted to each other, forming an ionic bond.
Properties of Ionic Compounds
- Ionic compounds are typically solids at room temperature.
- Ionic compounds are hard and brittle.
- Ionic compounds have high melting points and boiling points.
- Ionic compounds are soluble in water.
- Ionic compounds conduct electricity when dissolved in water or melted.
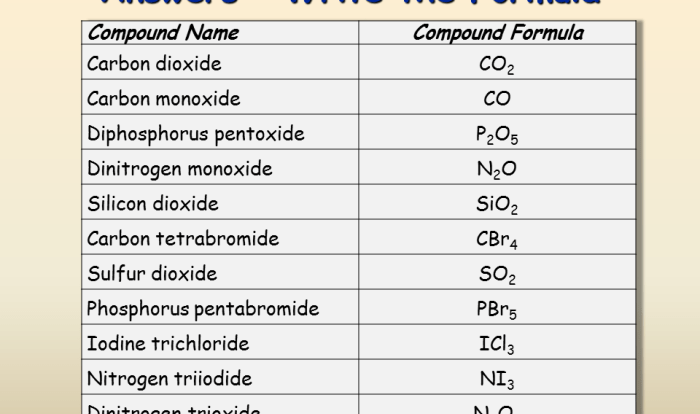
Examples of Ionic Compounds, Formation of ionic compounds worksheet
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium fluoride (CaF 2)
- Magnesium oxide (MgO)
- Aluminum oxide (Al 2O 3)
FAQ Guide: Formation Of Ionic Compounds Worksheet
What is the primary objective of the formation of ionic compounds worksheet?
To provide a comprehensive understanding of the formation, properties, and applications of ionic compounds.
How does the worksheet approach the topic of ionic compound formation?
Through a combination of theoretical explanations and interactive exercises, the worksheet guides learners through the process of ionic bond formation.
What are the key benefits of completing the formation of ionic compounds worksheet?
Enhanced understanding of ionic bonding, improved problem-solving skills, and a deeper appreciation for the role of ionic compounds in various fields.