Introducing the Lewis dot structure worksheet answer key, an indispensable tool for comprehending the fundamental principles of chemical bonding. This comprehensive guide provides a thorough understanding of Lewis dot structures, empowering students to excel in their chemistry coursework and research endeavors.
Delving into the intricacies of Lewis dot structures, this guide elucidates the concept, explores its significance in predicting molecular geometry and bonding, and showcases its practical applications in chemistry research.
Lewis Dot Structure
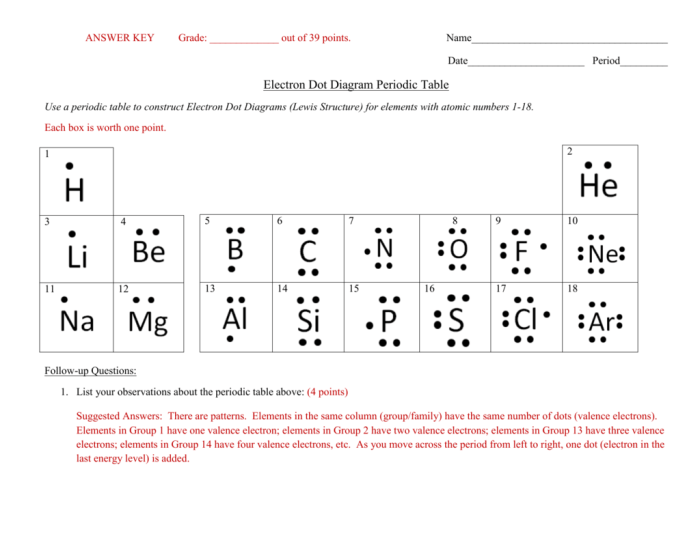
A Lewis dot structure is a diagram that represents the valence electrons of an atom or molecule. Valence electrons are the electrons in the outermost shell of an atom, and they are responsible for chemical bonding. Lewis dot structures are used to predict the shape and bonding of molecules, and to understand their chemical properties.
To draw a Lewis dot structure, first determine the total number of valence electrons in the atom or molecule. Then, place the electrons around the atom or molecule, two at a time, until all of the valence electrons have been used.
The electrons are placed in pairs, and they can be placed on the same side of the atom or molecule, or on opposite sides.
For example, the Lewis dot structure of hydrogen is H:, and the Lewis dot structure of oxygen is O:. The hydrogen atom has one valence electron, and the oxygen atom has six valence electrons.
The octet rule is a rule that states that atoms are most stable when they have eight valence electrons. This rule is followed by most atoms, but there are some exceptions. For example, hydrogen atoms are stable with only two valence electrons, and helium atoms are stable with only two valence electrons.
Lewis Dot Structure Worksheet

The following is a sample Lewis dot structure worksheet. Complete the worksheet by drawing the Lewis dot structures for the following elements and compounds:
- Hydrogen
- Oxygen
- Nitrogen
- Carbon
- Water
- Carbon dioxide
- Methane
- Ammonia
When drawing the Lewis dot structures, be sure to follow the octet rule. If an atom has more than eight valence electrons, the extra electrons will be placed in the next shell.
Answer Key for Lewis Dot Structure Worksheet
The following is the answer key for the Lewis dot structure worksheet:
- Hydrogen: H:
- Oxygen: O:
- Nitrogen: N:
- Carbon: C:
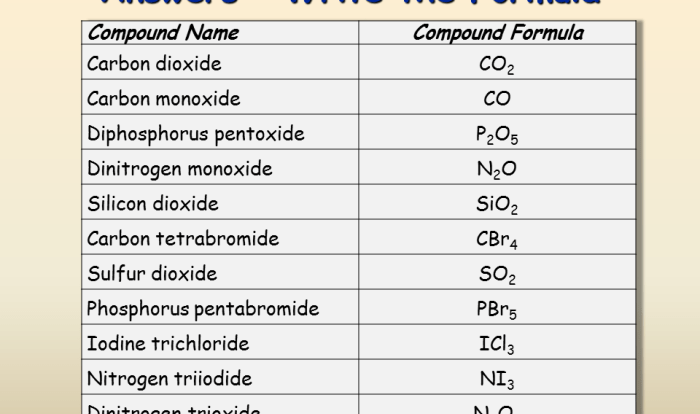
- Water: H-O-H
- Carbon dioxide: O=C=O
- Methane: H-C-H
- Ammonia: H-N-H
Note that the Lewis dot structure for water is an exception to the octet rule. Oxygen has six valence electrons, and each hydrogen atom has one valence electron. This gives water a total of eight valence electrons, but the oxygen atom has only two lone pairs of electrons.
This is because the oxygen atom is also bonded to two hydrogen atoms, which each have one lone pair of electrons.
Applications of Lewis Dot Structures
Lewis dot structures are used in a variety of applications, including:
- Predicting the shape and bonding of molecules
- Understanding the chemical properties of molecules
- Designing new molecules with specific properties
Lewis dot structures are a powerful tool for understanding chemistry. They can be used to predict the behavior of molecules, and to design new molecules with specific properties.
Resources for Learning Lewis Dot Structures
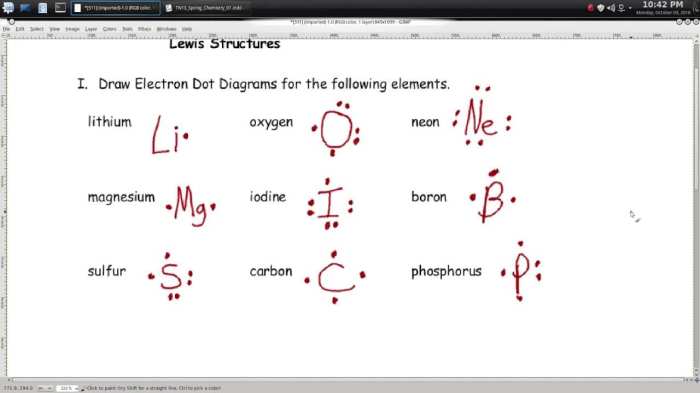
There are a number of online resources and tutorials available for learning Lewis dot structures. Some of these resources include:
In addition to these online resources, there are a number of textbooks and reference materials available on Lewis dot structures. Some of these resources include:
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy
- General Chemistryby Raymond Chang and Kenneth A. Goldsby
- Inorganic Chemistryby Catherine E. Housecroft and Alan G. Sharpe
Question Bank: Lewis Dot Structure Worksheet Answer Key
What is the octet rule?
The octet rule states that atoms tend to gain or lose electrons until they have eight valence electrons, resulting in a stable electron configuration.
How do I use the Lewis dot structure worksheet?
Follow the instructions provided in the worksheet to determine the number of valence electrons for each atom, arrange the atoms to minimize formal charges, and connect them with lines representing shared electron pairs.
What are the limitations of Lewis dot structures?
Lewis dot structures do not account for resonance, delocalization, or the three-dimensional nature of molecules.