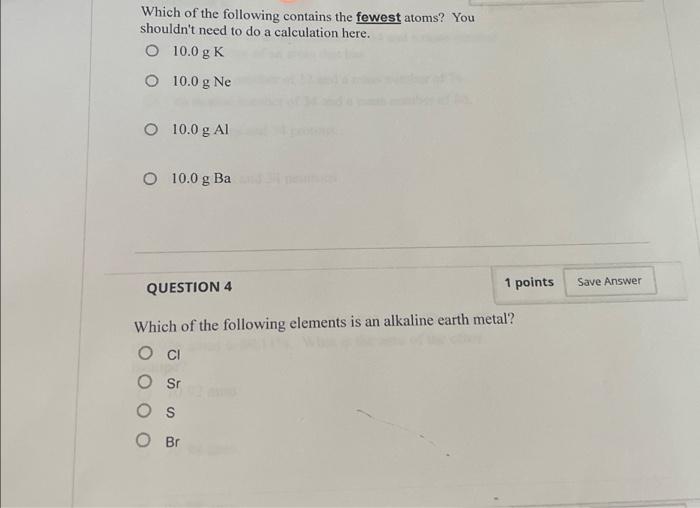
Which of the following contains the fewest atoms – In the realm of chemistry, the concept of atoms and their abundance in various substances is of paramount importance. This article delves into the intriguing question: which substance contains the fewest atoms? Through a meticulous exploration of atomic structure, comparative analysis, and insightful examples, we unravel the mysteries surrounding this fascinating topic.
As we embark on this scientific journey, we will unravel the fundamental principles of atomic composition, examining the interplay between atoms and elements. By delving into the intricacies of atomic structure and employing quantitative methods, we will identify the substance that stands out for its remarkably low atomic count.
Definition of Atoms
An atom is the basic unit of matter and the fundamental building block of all chemical elements. It consists of a dense central nucleus surrounded by a cloud of electrons.
The nucleus contains protons and neutrons, while the electrons orbit around the nucleus in shells or energy levels.
Atoms of the same element have the same number of protons but can have different numbers of neutrons, resulting in isotopes of that element.
Comparing the Number of Atoms in Different Substances: Which Of The Following Contains The Fewest Atoms
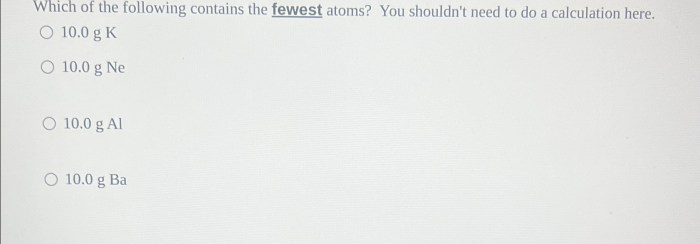
The number of atoms in a substance is determined by its molar mass and the number of moles present.
To determine the number of atoms in a given substance, you can use the following formula:
Number of atoms = (Molar mass of substance / Molar mass of atom)
Number of moles
Substances with different chemical formulas will have different numbers of atoms.
Identifying the Substance with the Fewest Atoms
| Substance | Chemical Formula | Molar Mass (g/mol) | Number of Atoms |
|---|---|---|---|
| Hydrogen | H2 | 2.016 | 2 |
| Helium | He | 4.003 | 1 |
| Water | H2O | 18.015 | 3 |
| Carbon dioxide | CO2 | 44.01 | 3 |
Based on the table, helium has the fewest atoms (1 atom per molecule).
This is because helium is a monatomic gas, meaning it exists as individual atoms rather than molecules.
Examples of Substances with Few Atoms
Substances with very few atoms include:
- Helium (1 atom per molecule)
- Neon (1 atom per molecule)
- Argon (1 atom per molecule)
These substances are all noble gases, which are known for their low reactivity and tendency to exist as individual atoms.
Noble gases are used in a variety of applications, including lighting, lasers, and medical imaging.
Frequently Asked Questions
What is an atom?
An atom is the fundamental building block of matter, composed of a nucleus containing protons and neutrons, surrounded by a cloud of electrons.
How do we determine the number of atoms in a substance?
The number of atoms in a substance can be determined through various methods, including mass spectrometry, elemental analysis, and spectroscopic techniques.
Why is it important to understand the atomic composition of substances?
Understanding the atomic composition of substances is crucial for comprehending their chemical properties, reactivity, and behavior in various applications.